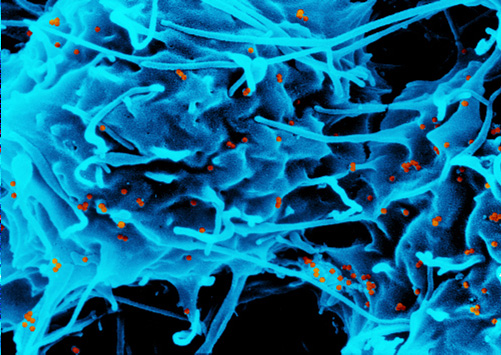
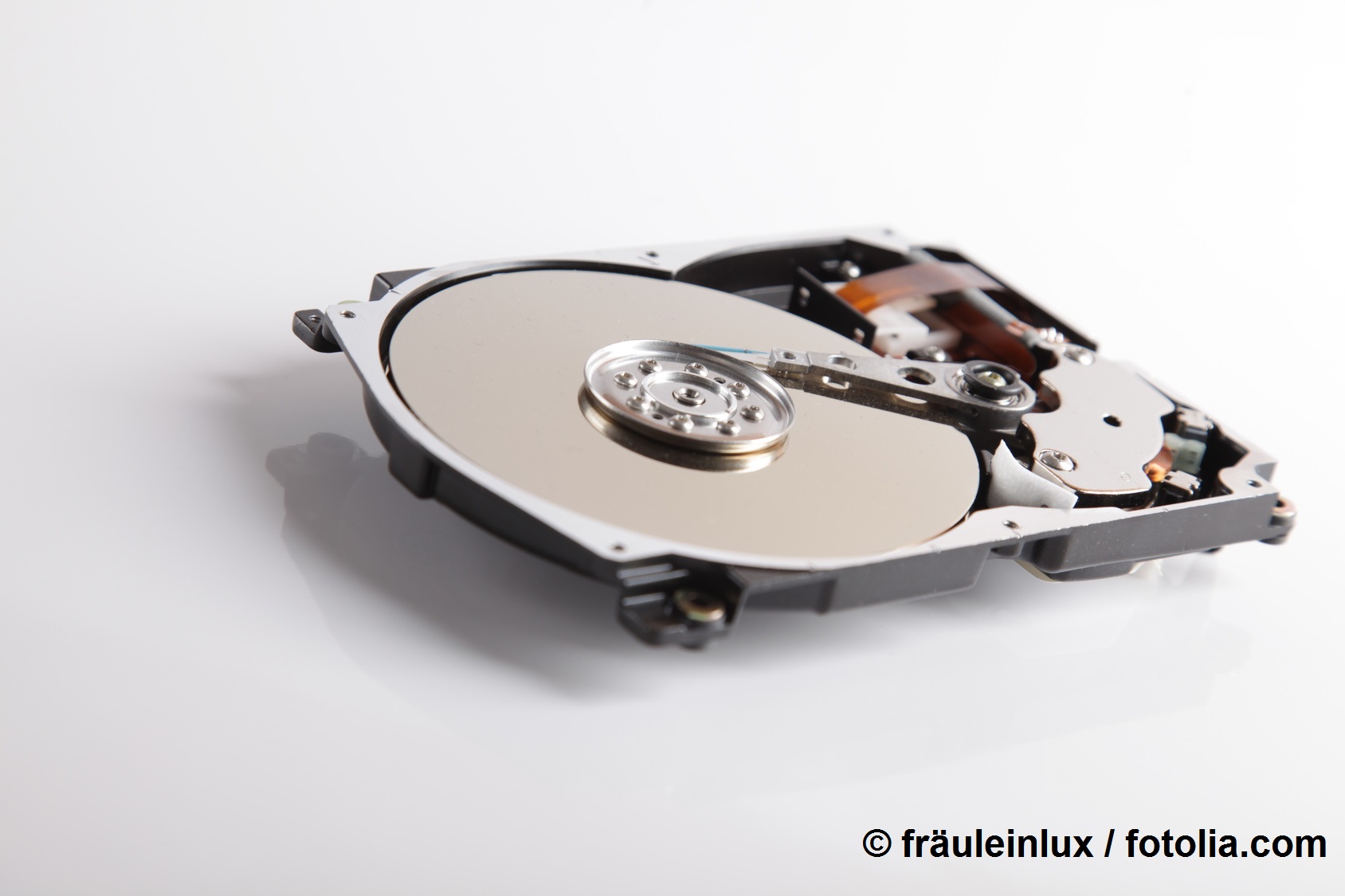
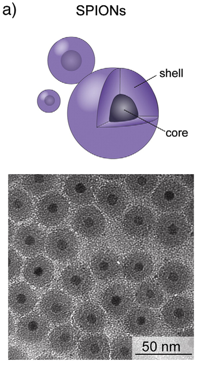
Iron is further processed into steel or electronic data storage media. Iron compounds are also used in catalytic processes in the chemical industry. Furthermore, scientists are currently researching the use of iron oxide particles in medicine, e.g. as a contrast agent or for tumour therapy. Iron is one of the most abundant metals on earth. Its oxides are mined as iron ore.
 How can I come into contact with this material?
How can I come into contact with this material?
There is little chance for the user to come into contact with iron and iron oxide nanoparticles used in electronic devices and in data storage media. In the medical field, however, iron oxide nanoparticles are injected directly into the human body. Therefore it is extremely important to ensure that the used concentrations of the iron oxide preparations are non-toxic.
Is there any risk from this material to humans and the environment?
The administered amounts of iron and iron oxide used in medical applications are very small compared to the natural iron supplies within the body and are considered non-toxic. Naturally occurring iron oxides are ubiquitously found in the environment making it difficult to distinguish between the naturally occurring forms and synthetically produced iron oxide nanoparticles. Environmental engineers are using this material for environmental remediation purposes such as the removal of toxic compounds from ground water where the nanoparticles usually remain after the chemicals have been eliminated. In general iron and iron oxide nanoparticles are considered to be non-toxic to the environment and its inhabitants, only exceptionally high concentrations can be problematic. Nevertheless it is essential to differentiate between the effects of metallic iron and the respective iron oxides as the former cannot be taken up by organisms. Iron oxides however are important trace elements which can cause adverse effects in both humans and wildlife when administered in excessive amounts.
Conclusion
In the day-to-day life the human body is only exposed to very small amounts of iron nanoparticles or iron oxide nanoparticles which are considered generally to be non-toxic. Iron and iron oxide are naturally occurring materials in the environment.
By the way…
- Iron oxide is also used as colour pigment to create the typical rust red colour. It is a long-lasting dye that can been found in many ancient paintings.
- Pigeons and bacteria are known to store iron oxide particles within their bodies to enable orientation with regard to the earth’s magnetic field.

 In low concentrations, iron and iron oxide nanoparticles cause no negative effects in bacteria and even promote growth. However, high concentrations trigger negative effects and inhibit growth. One reason for the
In low concentrations, iron and iron oxide nanoparticles cause no negative effects in bacteria and even promote growth. However, high concentrations trigger negative effects and inhibit growth. One reason for the  Iron oxide nanoparticles accumulate on the body surface and in the intestine of water fleas and brine shrimps. Detrimental effects of high
Iron oxide nanoparticles accumulate on the body surface and in the intestine of water fleas and brine shrimps. Detrimental effects of high  High concentrations of iron oxide nanoparticles cause
High concentrations of iron oxide nanoparticles cause 
 In various fish species, iron and iron oxide nanoparticles also induce oxidative stress in high concentrations and cause stronger effects compared to iron salts, e.g. an increased mortality in zebrafish. The gill cells of rainbow trout and tilapia are not negatively affected by iron oxide nanoparticles.
In various fish species, iron and iron oxide nanoparticles also induce oxidative stress in high concentrations and cause stronger effects compared to iron salts, e.g. an increased mortality in zebrafish. The gill cells of rainbow trout and tilapia are not negatively affected by iron oxide nanoparticles. In fruit fly larvae, environmentally relevant (very low) concentrations of iron oxide nanoparticles cause no
In fruit fly larvae, environmentally relevant (very low) concentrations of iron oxide nanoparticles cause no  Iron oxide nanoparticles attach to the surface of green algae. The associated shadowing of the light leads to a reduced growth of the algae, since they depend on the sunlight.
Iron oxide nanoparticles attach to the surface of green algae. The associated shadowing of the light leads to a reduced growth of the algae, since they depend on the sunlight.