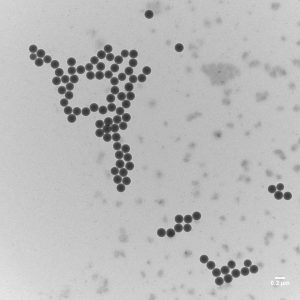
Polystyrene nanoparticles can be inhaled. They can also enter the body via food. Healthy skin, on the other hand, is a good barrier for these particles.
Uptake via the lung
The uptake of polystyrene nanoparticles via the lung may play a role primarily at the workplace. Nanosized plastic particles occurring in the environment can also be inhaled. However, the quantities of these secondary polystyrene nanoplastic particles are very low. Furthermore, there are approaches in medicine to use polystyrene nanoparticles as a means of transport for the administration of medicines via the lung.
In vitro experiments have shown that polystyrene nanoparticles are taken up by epithelial cells and macrophages of the lung, but do not cause any negative effects. In vivo, polystyrene nanoparticles administered by instillation also do not cause toxic effects or inflammatory reactions in rats. The immune system thus considers the polystyrene nanoparticles to be harmless.
In the alveoli, the inhaled polystyrene nanoparticles initially come into contact with the lung surfactant. This fluid is responsible for maintaining surface tension in the alveoli and is made up of 90% lipids and protein complexes with a large lipid content. These surfactant-protein complexes form a protein shell around the polystyrene nanoparticles, which usually has a mitigating effect on the potential effects of the nanoparticles. Studies on an in vitro infection model of lung cells have shown that the loading of the polystyrene nanoparticles with surfactant-protein complexes has a dose-dependent influence on the control of influenza viruses. Low concentrations of the loaded polystyrene nanoparticles inhibited the fight against influenza viruses, while higher concentrations promoted it. Whether this effect can also occur in the living organism is not yet known zotpressInText item="{4274171:BIXJKYFE},{4274171:G5L9CL49},{4274171:BV6L2TKT},{4274171:884S6UAI}"].
Uptake via the skin
Skincare and cosmetic products bring the skin into contact with larger polystyrene particles (not nano). Although polystyrene nanoparticles are not considered critical, they are not currently approved for use in cosmetic products in the EU.
If the skin is healthy, the polystyrene nanoparticles cannot overcome this protective barrier and enter the body (cf. body barriers – nanoparticles and the skin). It has been shown that polystyrene nanoparticles can penetrate hair follicles, but no effects could be demonstrated.
The question of how damaged or injured skin deals with nanoparticles is discussed again and again. An in vitro model of human corneal cells has shown that polystyrene nanoparticles can slow down wound healing in the case of injuries. However, it is not known whether this effect also occurs in reality for normal skin, i.e. on living organisms. .
Uptake via the gastro-intestinal tract
Technically produced polystyrene nanoparticles, as well as secondary polystyrene nanoparticles, can be taken up into the human body via food. They are considered uncritical, but there is a lack of data, especially on the long-term effects of secondary polystyrene nanoparticles.
Uptake via food is considered the most important uptake route for polystyrene particles in the human body. There are no data on nanoplastics in food, which is also due to the fact that detection and differentiation from other substances is hardly possible so far .
Studies on the uptake and effect of polystyrene nanoparticles have so far only been carried out with model particles. Such technically produced polystyrene nanoparticles have a uniform size, shape and purity.
A series of tests on chicken chicks showed that two weeks of administration of polystyrene nanoparticles impaired iron absorption in the intestine. Feeding polystyrene nanoparticles to rats over a five-week period resulted in no significant changes in animal behaviour in a series of tests .
There are currently no data on real long-term effects (over years) for animal models of human health. It is not known whether the particles remain in the intestinal lumen or will be taken up into the body via the intestinal barrier. There are animal studies that have shown an accumulation of polystyrene nanoparticles in Peyer’s patches, a collection of lymphoid follicles in the intestine. However, effects have not been described.
Uptake via medical application
The fact that polystyrene nanoparticles can be taken up by cells leads to their medical application, e.g., as carriers for other substances whose effect is improved by binding to particles. One new possibility for cancer therapy, for example, are the so-called "checkpoint inhibitors", for whose scientific discovery the Nobel Prize in Medicine was awarded in 2018. These substances do not target the tumour, but the immune system, which is induced to attack the tumour. In animal experiments, it has already been shown that the effect is significantly better when the drugs are bound to polystyrene nanoparticles (e.g. ). The majority of the analysed applications, like this one, focus on cancer. So far, polystyrene nanoparticles have not been used clinically, but efforts are underway in this direction, including for other diseases. A particularly interesting possibility for stable particles such as polystyrene nanoparticles is oral administration, because such particles survive the extremely acidic environment in the stomach well.
Polystyrene nanoparticles do not cause any toxic effects in the human body. However, they can impair the absorption of other substances in the intestine, such as iron. Long-term effects need to be analysed in more detail.