When talking about surface coatings of nanomaterials often terms such as modification, functionalisation or stabilisation are used. This diversity in terms reflects the different motivations for using a coating or for its intended function.
Generally, surface coatings of nanomaterials are applied in order to selectively change or influence distinct particle properties. For this purpose, the surface of a particle (the “core“) can be covered with a wide variety of substances which in return generates single- or multi-layers layer(s) (the “shell”) that can be either complete or incomplete. Examples for possible “shell”-substances are shown in the table below. As a result it is the shell rather than the core of the particle that determines the main final properties of the coated nanomaterial.
Compared to larger particles nano-scaled objects possess altered surface properties, especially if they are smaller than 10 nm. This is due to the large surface to volume ratio relating to the total surface area of the system exemplary shown in the figure below. These new properties are crucial for the behaviour of the particles during the different stages of production, processing and final application(s) as well as for possible interactions with the environment and humans.
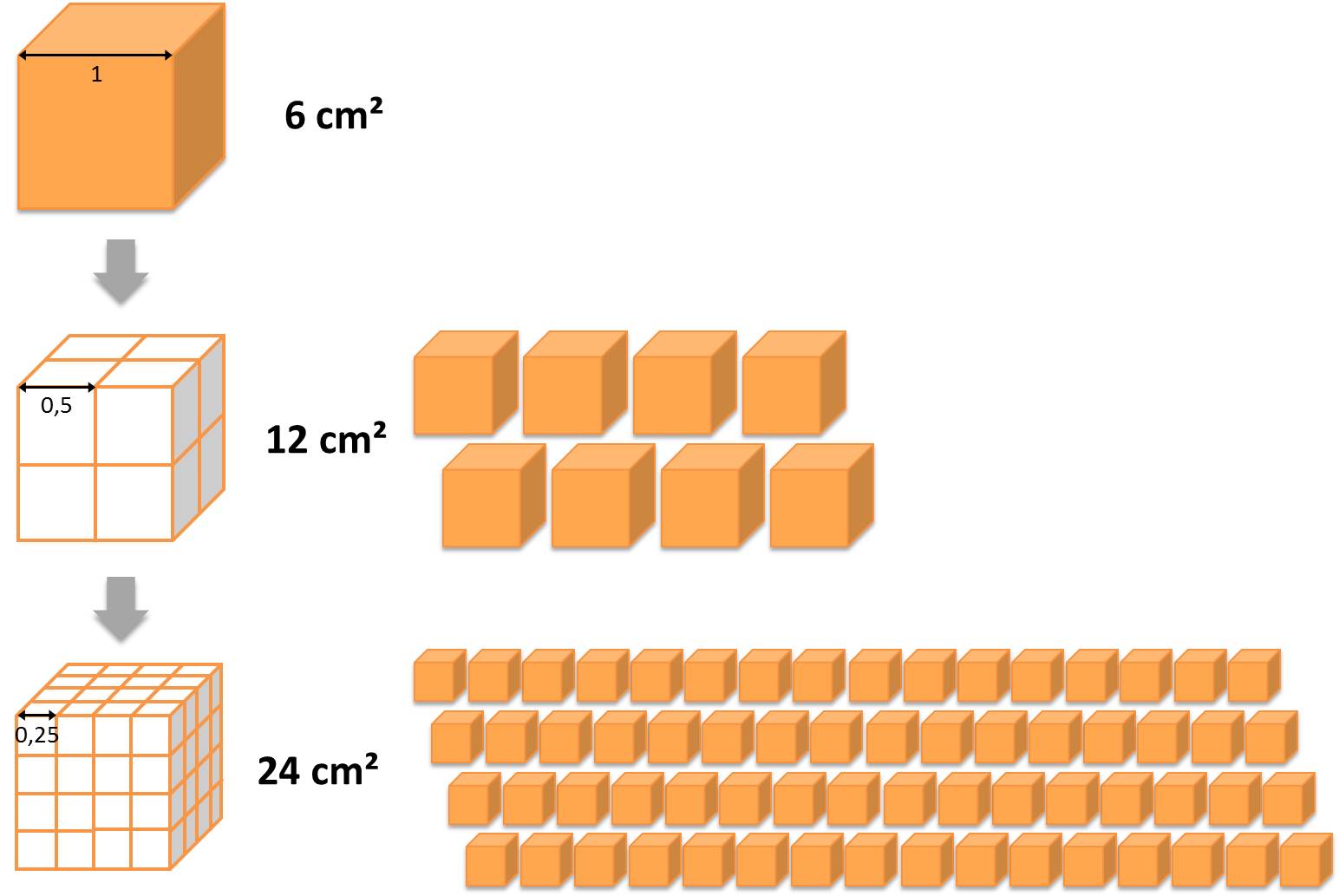
On the one hand the surface of a particle affects the physico-chemical behaviour of a nanomaterial (e.g. reactivity and solubility) or the biological properties such as compatibility with tissues (in medical applications). On the other hand, the surface also determines the level of interactions between the particle(s) and other molecules (e.g. proteins) and all kinds of different surfaces. For a desired particle coating all sorts of molecules (single molecules or groups), polymers as well as inorganic or metallic layers can be used. Due to the wide variety of surface coatings available worldwide, the nanoobjects’ properties can be tailored with regard to a specific application.
The following table gives an overview of possible types of coatings, their functioning and benefits.
| Role of the coating |
Coating material |
Remarks | Schematic images |
| (uncoated) | —- |  |
|
| Increased particle stability in solutions | Molecules, polymers (10 – 100 kDa), charged polymers (electrolytes, surfactants) |
Single layers, mostly used in production and processing |
 |
| Improvement of wettability | Molecules, polymers, inorganic layers |
To facilitate the preparation of mixtures of nanoparticles with water (hydrophilicity), or organic solvents and polymers (hydrophobic surfaces) |

|
| Prevention of particle core dissolution e.g. for silver (Ag) or zinc oxide (ZnO) | Inorganic layers, mostly silicon dioxide (SiO2) | Small particles have an increased solubility. The coating layer should help to maintain the chemical properties and to prevent the dissolution. |  |
| Improvement of physical and chemical functions, increased efficiency | Inorganic layers, mostly silicon dioxide or ZnO in combination with SiO2 | Fluorescence of quantum dots (QDs) can be significantly increased when saturating free surface bonds |  |
| Cost and material savings | Noble metals, e.g. palladium | Application of thin, incomplete layers of a noble metal catalyst on a cost-efficient substrate material (core) |  |
| Protective function | Organic layers | Protection of particle functionality, e.g. from catalyst poisons |  |
| Biocompatibility and functionality | Biocompatible polymers and inorganic layers, antibodies and peptides | Biocompatible SiO2 or Polyethylene glycol (PEG) can decrease protein adsorption, diagnostics and drug-transport |  |
The new surface properties may also affect potentially adverse effects or influence the environmental fate of particles. For some types of coatings adverse effects have already been reported [1,2]. Hence if a coating for nanomaterials is applied in a research study the coating has to be studied and assessed as well.
Coatings in manufacturing and processing
During the production, processing or for the final product most nano-objects are used in the form of a suspension having been dispersed in an (aqueous) medium. To stabilise such suspensions and to prevent sedimentation or agglomeration, molecules or polymers are linked to the particle surface.
In case of gold (Au) nanoparticles citric acid is often used, while polymers are well-suited for titanium dioxide (TiO2) particles. Another important property of nanoobjects is the wettability with water or organic solvents. This feature (wettability) can be adjusted and fine-tuned with specific coatings. The addition of so-called functional groups allows for controlled interactions with the solvent(s) and can hence be adapted to the respective needs.
Coatings to improve the nano-effect
The second important group of coatings has been designed to improve or strengthen the nano-effects. In particular, quantum dots (QDs), whose core is composed of cadmium selenide molecules (CdSe), require a coating. First of all CdSe – QDs are instable and dissolve subsequently. Secondly due to the surface defects of QDs the overall fluorescence is reduced. Therefore an intended coating for quantum dots has to meet certain chemical and physical requirements. It should be transparent to not affect the particle fluorescence and should also be able to provide for the desired long-term stability.
Coatings of nanoobjects for medical applications
The most complex and also most complicated coatings of nano-objects have been developed for medical applications, especially for diagnostic and therapeutic purposes. Besides iron oxide(s) also gold and quantum dots can be employed as core or carrier nanomaterials. Furthermore researchers are currently working on applications with coated nanomaterials based on silicon dioxide (SiO2) or carbon nanotubes (CNTs).
The desired or chosen coating has to fulfil the following criteria:
- first to ensure biocompatibility
- secondly a controlled residence time in the blood and
- thirdly the targeted transport to certain cells or organs.
By using appropriate antibodies which have been attached to the particle surface, the coated particles will be directed to target organs and cells like tumour cells or inflamed areas. The already existing biocompatible coating is further on used for the linking of the antibody.
Long residence time in the blood can be achieved with a surface coating of polyethylene glycol (PEG). In contrast to that short residence times and thus a more rapid absorption into the liver can be realised using dextran. Particles with such elaborate coatings can reach final diameters of 50-100 nm resulting in a coated particle that can be up to 5-10 times larger than the inorganic core (centre).With this course of action the resulting behaviour of the particles towards the cells is mostly determined by the composition of the coating and to a lesser extent by the original material particle (core).
Literature
- Zhang, L et al. (2011), Toxicol Lett, 207(1): 73-81.
- McGuinnes, C et al. (2011), Toxicol Sci, 119(2): 359-368.