A membrane (from the Latin membrana, meaning “membrane”) is a thin layer of one or more materials that separates the spaces on either side of the membrane and also influences the transport of substances across the membrane.
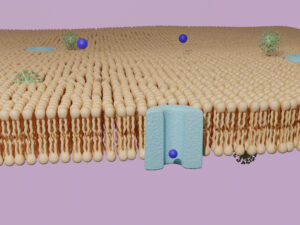
Every living cell is surrounded by a membrane, which has two functions: to separate the cell from its environment and to regulate the transport of substances into the cell (water, salts, vital substances) and out of the cell (metabolic products, waste products).
In addition to these biological membranes, there are also synthetic membranes. They are used in technical processes for separating or filtering substances, in applications as diverse as water treatment, chemical separation technology or medical technology, e.g. blood filtering in artificial kidneys.
What are the properties of membranes and what are they used for?
An important characteristic of membranes is their permeability. This can range from completely impermeable to semipermeable to omnipermeable.
The semi-permeable membrane can be thought of as a porous layer with a pore size that only allows water, solvents or other particles up to a certain size to pass through and is impermeable to larger particles. For example, biological cells have semi-permeable membranes that allow water to spontaneously pass from the side with lower salt concentration to the side with higher salt concentration. The greater the difference in concentration between the spaces separated by the membrane, the faster the water will pass through the membrane. The driving force is called osmotic pressure.
Technically, this process can also be reversed in reverse osmosis by mechanically pressing the side with the higher salt concentration against the membrane so that the pressure difference runs in the opposite direction. This process is used in water treatment or seawater desalination.
Another important case is the selectively permeable membrane, which is used in biological membranes to ensure that only certain substances (e.g. sodium or calcium) can pass (permeate) through the membrane. Selectivity is the ability of the membrane to distinguish between at least two components of a mixture. In contrast to the semipermeable membrane, it is not the size but the type of particle that determines whether it can pass through the membrane. In biological membranes, membrane proteins are often involved in active or passive transport processes, are highly selective and pass through pores.
In technical applications, membranes are used to separate mixtures of substances. Selectivity is expressed as the ratio of the permeability of the membrane to the different substances.
Economic importance
Energy consumption for separation processes accounts for about 10-15% of total energy consumption in industrialised countries. Membrane-based separation processes are attractive because they generally require only a fraction of the energy of other separation processes. For example, membrane separation of two liquids would use about 90% less energy than distillation, a separation process based on the differential evaporation of the components to be separated. The range of applications for technical membrane separation processes is very broad. Water treatment and demineralisation have already been mentioned, as have medical applications such as blood filtering and oxygen enrichment in heart-lung machines and lung support systems. In food technology, for example, membranes are used to separate milk components or to treat or filter liquids.
In the energy sector, membranes are essential components in batteries or in electrolysers for the production of green hydrogen. Gas separation applications range from the treatment of biogas to the purification of gases, the removal of CO2 from natural gas and the separation of air into oxygen and nitrogen. Finally, there is a wide range of applications in chemical engineering for the separation of products, by-products or impurities in chemical production processes.
Which materials are used for synthetic membranes and what do membrane modules look like?

Membrane materials can basically be divided into inorganic membranes and synthetic (polymeric) membranes. Inorganic membranes are made from ceramic materials, carbon or metals. Ceramic and carbon membranes can contain larger or finer pores depending on the application, the finest pores are only visible under special microscopes. Inorganic membranes usually have the advantage of high mechanical stability and good chemical resistance, even to aggressive chemicals such as acids or alkalis, or to organic solvents. They can also be used at high temperatures. However, the vast majority of membranes used in technical applications belong to the group of polymer membranes, for which low-cost and automated manufacturing processes allow the targeted adaptation of structures and properties. These materials are also easy to form and can be bonded or welded. On the other hand, the mechanical stability, chemical resistance and temperature range of polymer membranes are much more limited.
Technical applications in separation technology require membranes that are selective, highly permeable and as durable as possible. To achieve high throughput, thin membranes and large membrane surfaces are sought. Membrane layers can be 100 times thinner than a sheet of paper. To ensure that the membrane is sufficiently stable, the actual membrane layer is often applied to a stable support material, which can then be available in the required geometry, i.e. tubular or disc-shaped. Membranes can also be stacked in a module or wound into a hollow fibre membrane module.
Membrane applications have great potential for energy and resource efficient processes. The developments in membrane processes and materials pursued in projects under the MaterialNeutral funding programme can be found in our projects section.