Catalysis describes one of the most important basic principles of chemistry: with the support of an auxiliary substance called a catalyst, a chemical reaction proceeds faster, more energy-efficiently, or in a preferred direction than a non-catalysed reaction. Ideally, a catalyst is not consumed during the reaction.

Many catalytic reactions lead to products that would only be obtained in sufficient quantities with a catalyst. Or the catalyst favours the reaction to the desired product, reducing the formation of undesired products, which saves energy and raw materials.
In the chemical industry, catalytic processes often contribute to sustainable, environmentally friendly, and economical production processes. Catalysts also help shift chemical reactions in a favourable direction in treating waste and waste gases, producing undesirable by-products and waste products in smaller quantities and in some cases, producing less harmful by-products.
Catalytic processes also take place in nature and in humans, which is then called enzymatic catalysis, biocatalysis or, in a special case, whole cell biocatalysis, see our article on biocatalysis. They are also used in industry. Without these biological catalytic processes, there would be no life on Earth.
Car exhaust catalytic converters
The best-known example of a catalytic process is the car exhaust catalytic converter, which significantly reduces the amount of pollutants in the car exhaust. In addition to influencing other undesirable reactions, these catalytic converters reduce the reaction of nitrogen to nitrogen oxides, which occurs preferentially in the exhaust gas without a catalytic converter. Depending on the type of engine and catalytic converter, the nitrogen oxides are reduced either by other exhaust gas components or by urea solution (AdBlue®) with the help of a precious metal catalytic converter so that only nitrogen is emitted in the car exhaust. This prevents the car from emitting nitrogen oxides, which react with water to form nitric acid and other nitrogen acids and cause acid rain, thus contributing to tree death.
It is known that catalytic converters release traces of the precious metals in car exhaust during use, e.g. platinum and palladium, which then end up in the environment, especially on the side of roads. So far, however, researchers have not found evidence that the minimal quantities of such precious metals cause damage to the environment. Even if any negative effects are identified in the future, such effects must also be considered in comparison to the massive environmental damage caused by car exhaust fumes through acid rain if car exhaust catalysis is not used.
Catalysis in the chemical industry

In the chemical industry, catalysts help make most processes (~80 %) for producing chemicals and materials more energy and resource efficient. Catalytic processes are, therefore, essential tools for chemists. They save the industry money, reduce environmental pollution and are key tools for a sustainable society.
One of the most important catalytic syntheses in chemistry is the Haber-Bosch process for ammonia production. In the Haber-Bosch process, ammonia (NH3) is produced by the direct reaction of hydrogen (H2) and nitrogen (N2) with the aid of an iron oxide-based catalyst. Without a catalyst, it is not possible to achieve an economically viable yield from the reaction. On the other hand, the catalytic Haber-Bosch process enables the current production of ammonia on a scale of millions of tonnes. Ammonia is converted into fertiliser, which ensures food supply in most countries. However, the catalytic ammonia production still requires higher temperatures (e.g. 400 °C) and high pressures (e.g. 200 bar).
Although this catalytic reaction made industrial ammonia production possible in the first place, nature can do better: it has developed systems that produce ammonia at room temperature (20° C) and atmospheric pressure (1 bar) in bacteria on the roots of legumes (pulses such as peas), with the help of nitrogenase enzyme. Ammonia compounds are used to fertilise plants. However, reproducing these natural catalytic reactions in artificial systems has not yet been possible. This impressive example shows why intensive research is still invested in catalytic processes. There is still a lot of potential for improving catalytic systems.
Catalyst types and components
Catalytic processes are widely used in chemical synthesis. The following list gives an idea of the range of metals used as catalysts (strongly simplified based on [1]). Depending on the desired reaction, different catalysts are used, and frequently, several catalysts promote the same reaction:
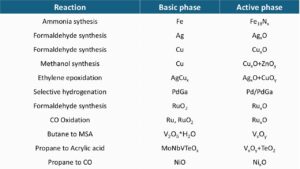
Automotive catalytic converters contain platinum, palladium, and rhodium precious metals. The figure shows, that the chemical industry uses many metals, including precious metals, in catalytic processes. As precious metals are especially expensive and some metals can be harmful to humans and the environment (e.g. cobalt or chromium), researchers are trying to reduce the amount of metals used in catalysts and replace precious metals wherever possible. In many cases, this also makes sense from a sustainability perspective, for example, if the precious metals required come from mines in countries with inadequate safety standards in labour protection or child labour.
The BMBF also supports research in the field of catalysis in the MaterialNeutral funding program, as this can save large quantities of important resources such as energy and raw materials.
Literarture:
- Schlögl, R (2015), Angewandte Chemie, 127(11): 3531-3589.